
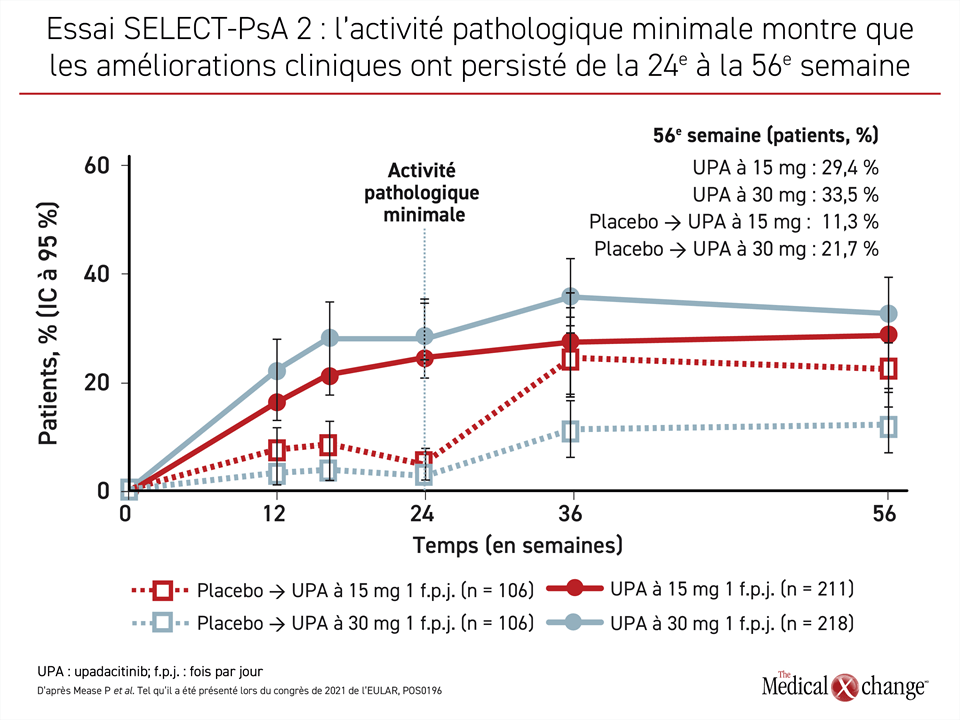
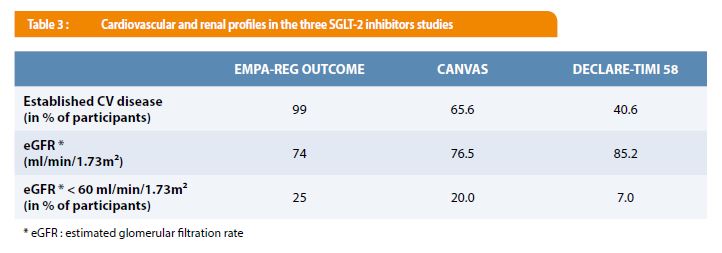
“There should be really no major safety concerns to go upstream and really try using these therapies early on after an event,” he said. It would be premature to use the DECLARE-TIMI 58 findings to state that the SGLT2 inhibitors are effective for primary prevention, but the potential for these drugs in expanded secondary prevention scenarios-both in diabetics and nondiabetics-is provocative, Udell said, adding that these drugs could be attractive options early after a cardiovascular event. “I think this demonstrates and kind of seals the deal that there is a class effect for at least heart failure, at a minimum, and likely for composite endpoints that have a mechanism of hemodynamic improvement,” Udell said. What is reassuring, he said, is that the impact on heart failure hospitalization has been consistent with all three SGLT2 inhibitors. A little bit of each of those factors could have played into the dapagliflozin results. The risk of moving to a lower-risk group in a trial, Udell said, is that it can be more difficult to demonstrate a drug effect over the same length of follow-up, there could be a different mechanism of risk in that group, or the level of risk could be too low to see an effect. DECLARE-TIMI 58 had the largest primary prevention cohort out of the three trials CANVAS mostly involved secondary prevention and EMPA-REG OUTCOME was all secondary prevention.
#DECLARE TIMI 58 TRIAL#
“And we think these data from DECLARE-TIMI 58 extend the benefit to a broader population of patients for primary and secondary prevention.”Ĭommenting for TCTMD, Jacob Udell, MD (University of Toronto, Canada), said the reason dapagliflozin did not reduce MACE in this trial like the other SGLT2 inhibitors did in their respective trials boils down to study design. “They have moderate benefits on atherosclerotic MACE that appear confined to those with established disease, but they have robust effects on reducing the risk of heart failure and renal outcomes which do not appear dependent on baseline factors,” he said. Taking into consideration results from the CANVAS trial program involving canagliflozin (Invokana Janssen), the EMPA-REG OUTCOME trial of empagliflozin (Jardiance Boehringer Ingelheim/Eli Lilly), and now DECLARE-TIMI 58, Wiviott said at a press conference that there are some conclusions that can be drawn about SGLT2 inhibitors as a class. Topline results were released last month. Stephen Wiviott, MD (Brigham and Women’s Hospital, Boston, MA), reported the findings, which were published simultaneously online in the New England Journal of Medicine, here at the American Heart Association (AHA) 2018 Scientific Sessions. The other co-primary endpoint, a composite of CV death or hospitalization for heart failure, was significantly reduced with dapagliflozin (4.9% vs 5.8% HR 0.83 95% CI 0.73-0.95), driven entirely by a relative 27% reduction in the risk of heart failure hospitalization (HR 0.73 95% CI 0.61-0.88). MACE (CV death, MI, or ischemic stroke), one of the efficacy endpoints, occurred in 8.8% of patients treated with dapagliflozin and 9.4% of those who received placebo (HR 0.93 95% CI 0.84-1.03), a difference that met criteria for noninferiority but not superiority. CHICAGO, IL-DECLARE-TIMI 58, a trial of the selective sodium glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga AstraZeneca) in patients with type 2 diabetes and high cardiovascular risk, met only one of its two co-primary endpoints, but it was still seen as supportive of the benefits of the SGLT2 inhibitor class for reducing heart failure risk.


 0 kommentar(er)
0 kommentar(er)
